產品中心
產品資料
產品名稱: pGL4.33[luc2P/SRE/Hygro]
產品型號:
產品展商: HZbscience
產品文檔: 無相關文檔
簡單介紹
pGL4.33[luc2P/SRE/Hygro]的各批次質粒菌株發貨前均經過嚴格的多重驗證,如存在質量問題,請在收到產品的三個月內通知我司。收到pGL4.33[luc2P/SRE/Hygro]后請短暫離心,取2μl轉化至對應感受態中,挑取單克隆重新提取質粒后使用。
pGL4.33[luc2P/SRE/Hygro]
的詳細介紹
pGL4.33[luc2P/SRE/Hygro]載體基本信息
| 載體名稱: | pGL4.33 ; pGL4.33[luc2P/SRE/Hygro] |
|---|---|
| 質粒類型: | 信號通路報告載體;哺乳動物載體;螢火蟲熒光素酶報告載體 |
| 高拷貝/低拷貝: | -- |
| 克隆方法: | 限制性內切酶,多克隆位點 |
| 啟動子: | Minimal Promotor |
| 載體大小: | 6112 bp |
| 5' 測序引物及序列: | -- |
| 3' 測序引物及序列: | -- |
| 載體標簽: | luc2P |
| 載體抗性: | 氨芐青霉素 |
| 篩選標記: | 潮霉素(Hygromycin) |
| 克隆菌株: | TOP10等常規菌株 |
| 宿主細胞(系): | HEK293等 |
| 備注: | pGL4.33[luc2P]載體是信號通路報告載體;含SRE應答元件;含螢火 蟲熒光素酶報告基因luc2P。 |
| 產品目錄號: | E134A |
| 穩定性: | 穩表達 |
| 組成型/誘導型: | 組成型 |
| 病毒/非病毒: | 非病毒 |
pGL4.33[luc2P/SRE/Hygro]載體質粒圖譜和多克隆位點信息
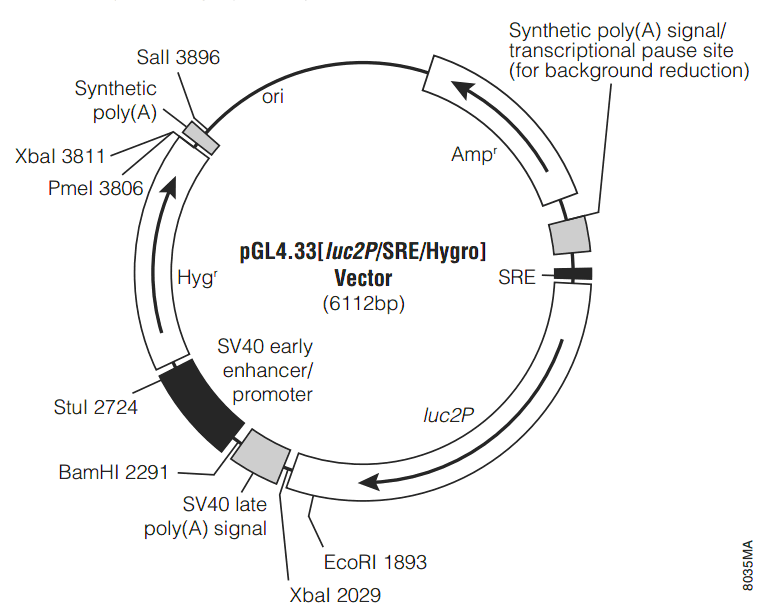

pGL4.33[luc2P/SRE/Hygro]載體簡介
The pGL4.33[luc2P/SRE/Hygro] Vector(a–c) contains a Serum Response Element (SRE) that drives transcription of the luciferase reporter gene luc2P in response to activation of MAPK/ERK signaling pathway. luc2P is a synthetically derived luciferase sequence with humanized codon optimization. The luc2P gene also contains hPEST, a protein destabilization sequence. The protein encoded by luc2P responds more quickly to induction than the protein encoded by the luc2 gene. The vector backbone contains an ampicillin resistance gene to allow selection in E. coli and the mammalian selectable marker for hygromycin resistance. Sample Protocol to Determine Induction of Luciferase by FBS + PMA in HEK293 Cells Transfected with the pGL4.33[luc2P/SRE/Hygro] Vector 實驗材料 Dulbecco's PBS (DPBS) 0.05% (w/v) trypsin in DPBS DMEM DMEM supplemented with 0.5%, 10% and 40% fetal bovine serum (DMEM/FBS) Phorbol 12-myristate 13-acetate (PMA, Promega Cat.# V1171 or Sigma Cat.# P8139), 1mg/ml solution in DMSO ONE-Glo Luciferase Assay System (Cat.# E6110) HEK293 cells transfection reagent Day 1: Plate Cells 1. Grow HEK293 cells in DMEM/FBS to approximately 75% confluency. 2. Harvest cells via trypsinization: Remove the DMEM/FBS, wash the cells with DPBS and add the trypsin/DPBS (1X volume). After 2 minutes, add a 4X volume of DMEM/FBS, collect the cell suspension and pellet the cells by centrifugation. Aspirate the supernatant, and resuspend in DMEM/FBS. We have routinely used a concentration of 10,000–15,000 viable cells/100μl DMEM/FBS. 3. Dispense 100μl of the cell suspension into the wells of a 96-well plate. Plate enough wells to perform each test condition in triplicate. 4. Cover the plate, and place it in a tissue culture incubator at 37°C overnight (or for 24 hours). Day 2: Transfect Cells 1. Transfect the cells using a high-efficiency transfection reagent. Each well of cells in a 96-well plate requires 0.1μg pGL4.33[luc2P/SRE/Hygro] Vector DNA. Transfection conditions may require optimization. 2. Cover the plate, and place it in a tissue culture incubator at 37°C. 3. After 4–6 hours, change the medium to DMEM/0.5%FBS (100μl per well) to start serum starvation. Day 3: Induce Transfected Cells 1. Prepare 2X induction and 2X control solutions. Calculate the volume of 2X induction and 2X control solution by multiplying the number of wells needed for each solution by 50μl, and prepare 110% of this amount. 2X induction solution: 40%FBS plus 20ng/ml PMA in DMEM 2X control solution: DMEM 2. Remove 50μl of medium from wells that will be treated with either 2X induction solution or 2X control solution. 3. Add 50μl of 2X induction solution to the cells to be induced and 50μl of 2X control solution to the control noninduced cells. 4. Return the plate to the tissue culture incubator, and induce for 6 hours. 5. Analyze luciferase activity using an appropriate luciferase detection assay. We have observed comparable results for fold induction of the vector using a variety of luciferase reagents, including: Bright-Glo Luciferase Assay System (Cat.# E2610, Technical Manual #TM052); ONE-Glo Luciferase Assay System (Cat.# E6110, Technical Manual #TM292); Dual-Luciferase Reporter Assay System (Cat.# E1910, Technical Manual #TM040); and Dual-Glo Luciferase Assay System (Cat.# E2920, Technical Manual #TM058). 6. Calculate the fold induction as follows: fold induction = average relative light units of induced cells average relative light units of control cells
pGL4.33[luc2P/SRE/Hygro]載體序列
hz-6348R PIWIL3 piwi樣3蛋白抗體hz-6349R VGF/Nerve growth factor inducible 神經生長因子誘導蛋白抗體
hz-6350R pan methyl Lysine 泛甲基賴氨酸抗體
hz-6351R DAP1 死亡相關蛋白1抗體
hz-6352R Adenylosuccinate Lyase 腺苷酸琥珀酸裂解酶抗體
hz-6353R HIPK2 Fas相互作用蛋白激酶2抗體
hz-6359R Glutamine PRPP amidotransferase 谷氨酰胺磷酸核糖基焦磷酸酰胺基轉移酶
hz-6365R CROT/COT 過氧化物酶體肉堿酰基轉移酶抗體
hz-6369R PAICS 磷酸核糖胺咪唑羧化酶抗體
hz-6370R p70 S6 kinase alpha 磷酸化核糖體p70 S6蛋白激酶抗體
hz-6371R Protamine 2 魚精蛋白2抗體
hz-6372R Hepcidin-25 鐵調節蛋白25抗體
hz-6373R CGR19 細胞生長調控蛋白19抗體(環指蛋白197)
hz-6374R FPR3/Lipoxin A4 receptor 甲酰肽受體3抗體(脂氧素A4受體)
hz-6375R CHMP1A 染色體修飾蛋白1抗體(金屬蛋白酶1)
hz-6376R DDIT4L DNA損傷誘導轉錄樣蛋白4抗體
hz-6377R DEAF1/Deformed Epidermal Autoregulatory Factor 1 畸形表皮自調節因子1抗體
hz-6378R EID3 E1A樣分化抑制因子3抗體
hz-6380R ERCC6L 發育相關蛋白ERCC6L抗體(乙醇致畸因子)
hz-6381R FAM107A 腎細胞癌下調蛋白1抗體
hz-6382R FRK/PTK5 胃腸道相關酪氨酸激酶抗體(蛋白酪氨酸激酶5)
hz-6383R GANP **中心相關核蛋白MCM3抗體
關于我們
產品中心
服務中心
Copyright@ 2003-2025
上海滬震實業有限公司版權所有
電話:021-60345367
傳真:021-31320307
地址:上海市楊浦區密云路1018號復旦科技園808室
郵編:200612
![pGL4.33[luc2P/SRE/Hygro]](http://y3.yzimgs.com/uploads/495330/2017717-143350948.png?imageView2/2/w/200/h/200|watermark/2/text/5LiK5rW35rKq6ZyH5a6e5Lia5pyJ6ZmQ5YWs5Y-4/font/5a6L5L2T/fontsize/300/fill/I0E3QTlBOA==/gravity/SouthEast)
